Reports: New Frontiers in Animal Care
Introduction
The value of innovation
Animal diseases and health challenges are constantly evolving, yet their impact on the global economy, human health and sustainable development remains underestimated.
Between 2000 and 2016, almost 360 animal disease outbreaks were recorded across 116 countries, causing enormous economic losses, impacting trade and affecting global food security. Of these outbreaks, two-thirds were caused by just five diseases, including Avian Influenza, Foot and Mouth Disease and African Swine Fever (ASF), highlighting the opportunities available to better target and manage livestock disease.
At the same time, pet ownership has skyrocketed across the globe. Over 50% of households in major markets currently own a pet and rises to over 80% in nations like Brazil and Argentina. This reflects the increased recognition that pets are not only companions but offer tangible benefits to our health and happiness. Owners are repaying that kindness by taking their pets to the veterinarian more often, offering the opportunity for better care that allows pets to thrive and support our lives.
Scientific advances and emerging technologies, from artificial intelligence to stem cell therapy and new generations of vaccines, have provided greater opportunities to predict, prevent, diagnose and treat animal illness more quickly, accurately and safely. Veterinary researchers and developers continue to break new ground in reducing disease spread and its impact.
The result is untold potential to improve animal welfare, protect livelihoods and increase efficiency; make raising livestock more sustainable; and offer longer, healthier lives for pets in our home.
Delivering sustainability
The health of animals, whether livestock or pets, is inextricably linked to the wellbeing of people and planet, meaning better animal health plays an integral role in sustainability. Healthier animals need fewer natural resources, allowing them to provide more food, labour, fertilizer, companionship and assistance for less feed, water and land. Their contribution will be central to efforts towards achieving the United Nation’s Sustainable Development Goals (UN SDGs) by 2030.
Innovations on the horizon in animal health offer the prospect of a world where the threat of disease is much reduced, thanks to stronger immunity, improved prevention strategies, earlier and more specific diagnosis, and more accurate and effective treatment. Bringing these innovations to fruition, from development to widespread availability, would mean lower emissions, less natural resource use, and fewer animals lost.
Yet for all the promise of growing veterinary knowledge and expertise, it is taking animal health companies increasingly longer and becoming more expensive to bring new products to the market.
Cost of innovation
Animal medicines and health products are necessary to prevent, diagnose and treat disease across over fifty different animal species. Developing innovations that are effective across this diverse landscape is a significant challenge, which is why a policy and regulatory environment that recognizes the unique characteristics of the animal health sector is essential.
A new medicine or product can take anywhere from five to 15 years from first discovery until it is available to treat an animal, and in Europe, this has increased by as much as two years since 2015.
Meanwhile, unless a product is patent-protected, the opportunity to recoup this investment is time-limited, particular in livestock. In Europe, companies have 10 years data protection once a new product receives a “market approval”. In the US, data protection lasts just five years, much of which can be absorbed by the global standard-setting process. For example, establishing Maximum Residue Levels (MRLs) for trade is necessary before a product can be used by producers who wish to export. This means by the time a product can be widely used, it may have already lost exclusivity protection and faces generic competition before the innovator can obtain a return on investment.
Furthermore, once a product receives a market approval, Good Manufacturing Practices (GMP) that are tailored to human health are often applied to the animal health sector. Without adapted GMP, an animal health product can face unnecessary requirements that may make it financially unfeasible to manufacture.
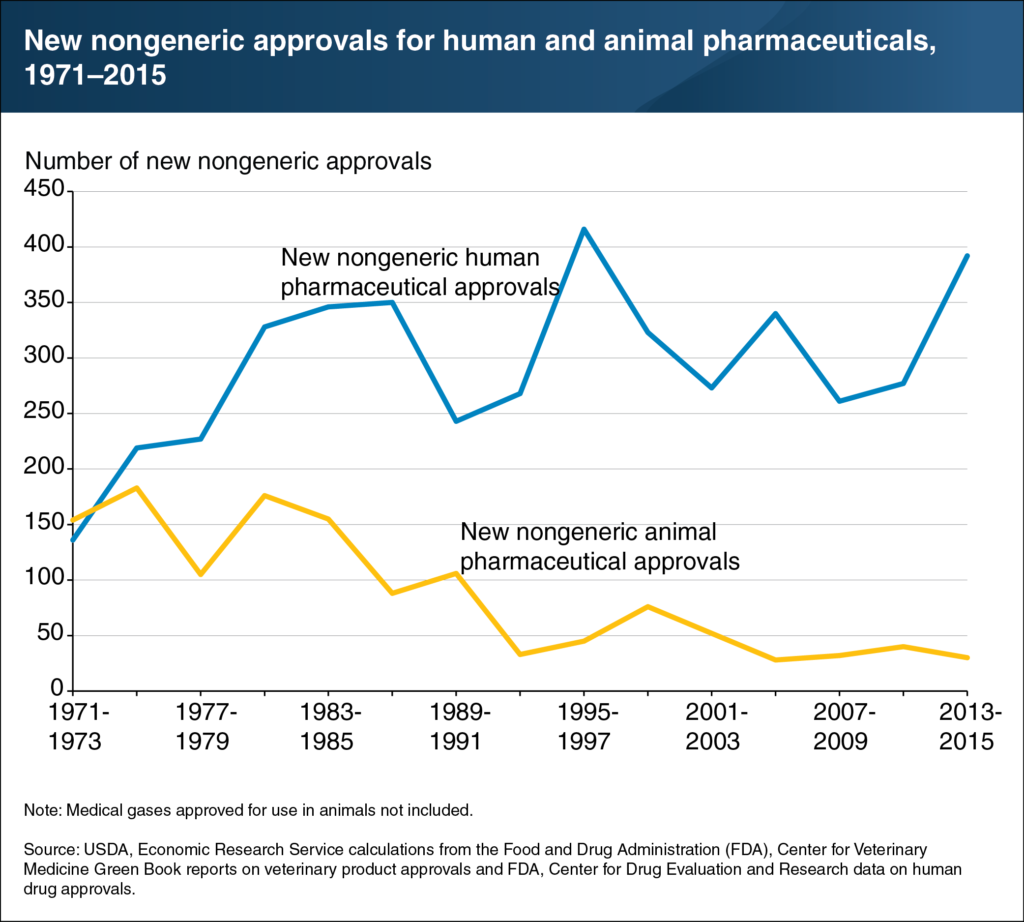
Altogether, this has contributed to a downward trend in the number of new approvals in some markets. In the early 1970s, U.S. approval rates for new animal products were on par with those for human medicine. While the rate of approval for human medicines skyrocketed to a peak of more than 400 by the mid-1990s, approvals in animal health have dwindled below 50.
Progress has been made in some markets to extend the data protection period of new products in recent years and introduce adapted GMP, improving the likelihood of a return on investment. However, hurdles such as the absence of a clear regulatory pathway and narrow criteria to qualify for data protection, continue to exist for any novel or emerging development that falls outside existing categories of products.
Despite the efforts of organizations such as VICH (Veterinary International Conference on Harmonization) to harmonize requirements, still many regions have widely differing regulatory standards. For example, the number of animals needed in trials to demonstrate safety and efficacy can vary, and manufacturers must satisfy the requirements for each market they intend to enter. For smaller markets, these regulatory costs can make it impossible for a company to recoup their investment once a product is approved, which means a company is unlikely to ever submit it for review. This ultimately limits the toolbox available to veterinarians, farmers and pet owners.
Finding ways to lift or overcome some of these barriers to innovation will be essential to fully unlock the benefits of innovation to animal health, and subsequently to the UN’s Sustainable Development Goals.
New vaccines
Overview
- Vaccines are among the most powerful tools in the veterinary toolbox, preventing disease to protect animals from later needing treatment and reducing losses.
- New vaccines and delivery methods provide opportunities to prevent more disease and protect more animals, offering a cost-effective way of safeguarding animal health and livelihoods.
- Vaccine development can be hampered by repetitive regulatory requirements that drive up costs and prolong the time needed for vaccines to reach market.
- Streamlining regulatory approval and assessment could inspire more and quicker vaccine development.
The state of innovation
A selection of technologies reaching the market or on the horizon
Vaccines are one of the most reliable and effective ways to prevent deadly animal diseases, helping protect animals, food supplies and livelihoods. Innovations in vaccine development can lead to new vaccines against previously deadly and costly diseases, as well as new ways to expand existing vaccinations to more regions and species.
mRNA vaccines
mRNA vaccines work by giving cells in the body a blueprint for how to fight off infectious disease. This recent breakthrough differs from traditional vaccines, which use an inactivated virus that triggers the immune system. mRNA vaccines instead provide a genetic code that act as instructions, showing the immune system how it can recognize and fight the disease (see graphic below). This safely equips the body to fight viruses without needing to use the virus itself. This means vaccines can be produced more efficiently, at lower costs and with fewer risks.
Timeframe: One to five years
Heat-resistant vaccines
Vaccines typically must be kept at cold, sometimes sub-zero, temperatures until they are administered to remain effective, which can hold back vaccination efforts in tropical countries or areas where refrigeration is not possible. Heat-resistant vaccines that remain effective at room temperature can help significantly reduce costs and improve animal health in developing regions. This has been achieved for vaccines against diseases including rinderpest and is being developed to protect against other infections.
Timeframe: Products are already on the market
Precision systems for vaccine delivery
New vaccine platforms can improve the efficiency and precision of mass vaccination. For example, systems to deliver vaccines to day-old chicks using a conveyor belt or inside the egg before hatching can improve vaccination rate among poultry. In aquaculture, oral vaccines and emerging automated injection systems allow for effective protection while avoiding manual injections that can be time-consuming.
Timeframe: Products are already on the market
‘Custom’ Vaccines
Autogenous vaccines offer farmers protection tailored specifically to their animals and situation. When a veterinarian recognizes that a herd is infected with a disease strain where standard vaccines may be ineffective, they can request a ‘custom’ or autogenous vaccine. Using a culture sample from the herd, the company will produce a vaccine targeted to that specific virus strain, which can help effectively protect uninfected animals on the farm and/or in nearby herds.
Timeframe: Products are already on the market
How an mRNA vaccine works
mRNA vaccines differ from traditional vaccines, and work by giving cells in the body a blueprint for how to fight off infectious proteins.
1. Messenger RNA (mRNA) are injected into the host containing instructions that teach cell how to create a key protein or ‘antigen’ from the target disease.
2. Example: Some human Covid-19 vaccines teach cells how to create just the ‘spike protein’ that is key to the disease.
3. Host cells follow the instructions and create the antigen.
4. Immune system detects the antigen and produces antibodies to fight it.
5. The host immune system now knows how to react when exposed to the disease in the future.
6. This mRNA approach can allow for faster, cheaper development of vaccines.
Sustainability benefits
More effective vaccines and delivery mechanisms can help protect more animals against diseases, which also means:
- Protecting the livelihoods of the millions worldwide who rely on livestock (SDG 1, 8 and 10);
- Reducing the need for antibiotics, which minimises the risk of antimicrobial resistance and helps protect public and environmental health (SDG 3, 15);
- Reducing the risk of zoonotic diseases passing from animals to people by preventing them in animals in the first place (SDG 3).
Barriers to innovation
- Repetitive approval submissions – in certain markets, a dossier of scientific evidence must be submitted for vaccines against each strain of a disease. This means manufacturers cannot respond quickly to new strains emerging in different regions. Similarly, new submissions are required for any change to a vaccine, even if it uses existing starting material.
- Inflexible field trials – testing a vaccine on animals relies on the continued presence of the disease. If a disease is seasonal or is no longer present, field trials can be delayed, holding up the approval process. By accepting new alternative testing methods, regulators could streamline this process.
- Lack of farmer incentive – it is difficult to generate a positive return on investment for vaccines that offer positive societal value, such as protection against zoonotic illness or reduction in carbon emissions, but little financial benefit to producers. This ultimately disincentivises research into vaccines that could benefit sustainability efforts.
Societal Value Challenge
Salmonella
Salmonella is one of the most common food-borne illnesses, and vaccinating poultry against it can better protect the public. However, farmers may see little productivity or income gains from the vaccine since poultry can harbour the disease but face no ill effects.
Emissions Reduction
Research is underway into tools such as vaccines or nutritional supplements that can reduce the amount of methane emitted by cattle. This can strengthen the environment, but farmers may not see any financial or production benefit.
Medicines that provide a public good but no direct gain for farmers can struggle to gain adoption in the market. This limits R&D opportunities in a valuable innovation space.
Opportunities to lift barriers to innovation
Vaccine and delivery platform development could be accelerated with greater regulatory flexibility.
- Vaccine antigen master file – increasing acceptance for a master dossier would allow for the singular approval of a basic vaccine technology, meaning subsequent assessments could focus on the novel or specific aspects of each vaccine submission.
- Multi-strain dossier – encouraging companies to submit a single dossier for a vaccine covering multiple strains would help increase the agility and flexibility to deal with different disease threats in different regions.
- Acceptance of alternative testing – by developing standards for new testing methodologies such as in vitro testing and eliminating unnecessary batch safety testing, regulators could increase the opportunities to demonstrate efficacy and safety while reducing the number of research animals needed.
- Producer incentives – incentives for producer adoption of vaccines that benefit society could provide a greater pull for research into innovative fields such as emissions reductions.
Alternatives to antibiotics
Overview
- Antibiotics are the cornerstone of modern medicine and public health protection against infectious disease. There are currently no alternatives to effectively treat bacterial infections.
- Antimicrobial resistance is a significant global threat to human and animal health.
- New compounds that offer a novel way to target bacteria without using antibiotics are among the most valuable potential innovations.
- Competition between human and animal health and a poor return on investment hold back innovation in developing alternatives.
The state of innovation
A selection of technologies reaching the market or on the horizon
True alternatives to antibiotics are products that target bacteria in a similar way and cure bacterial infection. Other innovations that reduce infection risk also reduce the need for antibiotics but cannot truly be called an alternative because they cannot treat a bacterial infection. Currently, antibiotics remain the only way to treat a bacterial infection. The animal health sector has taken significant action in recent years to improve responsible use while researching potential alternatives.
Bacteriophages
Bacteriophages – literally meaning “bacteria eaters” – are a type of virus that infects bacteria and destroys the host cells. Unlike antibiotics, bacteriophages are narrow in their application and target specific bacteria, therefore cannot be applied using a blanket approach. This is one reason why, although bacteriophages have been researched for decades, viable products have yet to reach the market. Like antibiotics, developments in bacteriophages that are also efficacious in people would likely be reserved for human medicine.
Estimated timeframe: 10 years or more
Ambient cold plasmas
Ongoing research into cold and ambient plasmas – or ionised gases – has shown that these substances can destroy harmful pathogens without leaving residues. A number of studies indicate that plasmas, which can be applied using a laser-like device to deliver a stream of plasma to a targeted area, could be used to prevent and treat microbial infections without damaging the tissue or posing a notable risk of causing antimicrobial resistance. Research suggests that the plasmas interfere with bacterial DNA and neutralise them. Cold and ambient plasmas could therefore reduce the need for antibiotics in treating chronic infections, and could also be used to decontaminate environments and improve biosecurity.
Estimated timeframe: One to five years
Antimicrobial peptides
Antimicrobial peptides – or AMPs – are broad-spectrum antimicrobial molecules that are produced in almost all animals as part of the immune system. In recent years, AMPs have become one of the most widely researched alternatives to conventional antibiotics due to their potency. AMPs could combat microbes and diseases without a significant risk of causing resistance.
Estimated timeframe: Five to 10 years
Nanotechnology
Treatments using nanotechnology – or tiny particles – for antibiotic-resistant bacteria are in development and test trials. Nanoparticles delivering antibacterial substances, such as propolis, a compound made by bees to protect their hives, could be added to animal feed to help treat infection. Such treatments could be customized for specific species and herds as an effective alternative to using antibiotics.
Estimated timeframe: Five to 10 years
Immunotherapies
Treatments that direct the immune system response could harness an animal’s natural defences against a bacterial infection. Immunotherapies are well known in human health fields such as cancer treatment, where these treatments are used to direct the immune system to attack certain cells or infections. Research is underway to create a better understanding of the livestock immune system and how a similar response could be elicited for bacterial disease.
Estimated timeframe: Five to 10 years
Sustainability benefits
Developing new products that prevent or treat bacterial infection while reducing the burden on antibiotics offer benefits for animal health and wellbeing, as well as:
- Improving efficiencies in animal agriculture, which generates greater income for farmers (SDG 1 and 8) and produces more food for the global supply chain (SDG2);
- Reducing the risk of antimicrobial resistance, which strengthens global public health (SDG3);
- Reducing the potential impact of antibiotic use on the environment (SDG12).
Barriers to innovation
Obstacles holding back new antibiotic alternatives can be significant:
- Limited possibilities – any new antimicrobial treatments that are efficacious in people are considered “critically important” and are reserved for human use only. This limits the pool of potential molecules for animal health research.
- Inflexible regulations – Antibiotic alternatives are typically assessed as conventional antibiotics because regulatory processes have not adapted to assess new and emerging categories of products.
- Broken market – it can take years and significant R&D to develop a new antibiotic. Yet if a new treatment does succeed, it may be treated as a drug of last resort and used sparingly to minimise resistance risk. Many companies are reluctant to invest in this process as it is near impossible to see a return.
- Limited patent protection – once companies patent a new molecule and are granted protection over this intellectual property, they are likely to spend much of this period bringing it to market. After this, there may be little time remaining to recoup the investment before the patent protection expires.
- Non-tariff trade barriers – some existing antibiotics have approvals but lack internationally accepted Maximum Residue Levels (MRLs) that enable use. As a result, some companies are concerned that it may be difficult to secure MRLs for novel antibiotics alternatives, which will limit farmers ability to use them and disincentivises companies from investing.
Opportunities to lift barriers to innovation
It is in the interests of the whole public health sector that human and veterinary medicine unite to address the issue of antibiotic resistance and the need for alternatives. This can be supported through:
- Expanded IP protection – an opportunity to support innovation in antibiotic alternatives lies with legislators, which could extend data exclusivity protection for these products.
- New market paradigms – while longer protection periods can offer greater financial incentives, government-run schemes could also be considered to secure future developments by guaranteeing a return on investment on new developments with limited market potential.
- More public investment – significant public resources have been invested in developing new treatments for bacterial infections in people to combat resistance. Animal health faces similar pressure but receives a small fraction of the public investment seen in human health. Greater parity could help generate new alternatives to using antibiotics in animals and better understandings of AMR transfer pathways.
- Product label claims – Considering reduction of antibiotic use as a clinical endpoint or efficacy measure as an allowable claim on product labels could help more innovations reach the market. This is not allowed under current guidelines and may sideline valuable products.
Reducing the need for antibiotics
Innovation across the entire animal health sector contributes towards tackling the issue of antibiotic resistance because reducing the risk of disease means reducing the need for antibiotics.
In 2019, HealthforAnimals and Members outlined its commitment to reducing the need for antibiotics. The industry pledged to invest at least $10 billion in R&D by the year 2025, as well as delivering at least 100 new vaccines, 20 new diagnostic tools, 20 new nutritional products, and 30 other products that can reduce the need for antibiotics and also outlined 50+ ways they are strengthening responsible use.
Download the Roadmap at HealthforAnimals.org/Roadmap

Digital technologies
Overview
- Digital technology offers opportunities for veterinary medicine to become more efficient and precise, enabling an individual-level of veterinary care to large groups of animals.
- Automation, greater connectivity and more health data can allow earlier diagnosis and more accurate treatment, but it also requires infrastructure and expertise.
- Greater levels of data also raise issues of confidentiality, ownership and capacity for adequate analysis.
- Regulators and governments can support the digital health revolution by working with developers to streamline regulations and considering appropriate incentives.
The state of innovation
A selection of technologies reaching the market or on the horizon
Advancements in digital monitoring and surveillance are rapidly transforming the animal health landscape, bringing improvements in speed and efficiency that enable individual-level treatment even in groups of hundreds or thousands of animals.
Sensors, Tags and Collars
New technology can allow farmers and veterinarians to treat herds at an individual level at a scale that is near impossible when relying solely on farm staff. For instance, sensors or smart tags on cattle can monitor activity, feeding, temperature, behavior and more. Reports and real-time alerts then allow animal caretakers to detect health issues in a single animal before they affect the rest of the herd. In pets, smart collars help owners continuously monitor health data that can be used to optimise feeding and care.
Timeframe: Products are already on the market
Real-time monitoring
Surveillance using cameras, microphones and sensors can produce accurate, continuous data demonstrating animals’ wellbeing, productivity and performance. Sound monitoring systems can catch the first signs of disease on fish farms based off the volume and frequency of feed consumption or in a swine pen by hearing a sick animal cough amongst the cacophony of sounds. In a cattle herd, thermal cameras can see the first elevated temperature amongst an entire cattle herd. These innovations allow for earlier diagnosis and targeted treatments, leading to improved animal health and reduced costs.
Timeframe: Products are already on the market
Prediction technologies
Software systems can harness the wealth of data provided by digital technologies to predict disease or health challenges before they occur. Through approaches like machine learning technologies and A.I.-driven algorithms, companies are developing tools that can analyze the continuous on-farm data, predict likely health threats and allow animal caretakers to take preventative measures. In some cases, tools can indicate a high ‘probability of disease’ that could allow for earlier treatment with the right regulatory framework.
Timeframe: Products are already on the market
Building design
The smart design of animal accommodation such as pens and barns can both improve animal health and incorporate monitoring and surveillance. High negative air pressure, similar to clean rooms in hospitals, can create a controlled environment while systems to monitor heat and ventilation can also ensure optimum conditions.
Timeframe: Products are already on the market
Monitoring Health
Sensors such as ear tags and smart collars can provide constant, real-time evaluation of livestock in areas such as:
Temperature: Sensors will monitor the animal’s temperature to detect the first signs of a fever.
Movement: GPS tracking can indicate if an animal is moving slowly or favouring one side, indicating lameness or onset illness.
Feeding: Measuring how much an animal is eating can show when appetite falls and disease may be settling in.
Sustainability benefits
Early disease detection and individual treatments can help support sustainable development by:
- Reducing costs and environmental impact associated with sick animals and supporting productivity (SDG 1, 2 and 8);
- Optimising labour and creating new opportunities for workers and youth (SDG 1 and 8);
- Enabling early diagnosis and treatment, which reduces the need for antibiotics and helps protect public health (SDG3).
Barriers to innovation
Digital tools have the potential to revolutionise animal agriculture if barriers can be lifted or overcome, including:
- Limited capacity to act upon new levels of data – while digital tools can generate significant data, farmers also to be able to aggregate, analyse and identify how to respond. In many regions, the agricultural staff required can be hard to find and retain, while rural connectivity is often a restricting factor for digital innovations.
- Lack of acceptance for probability – with more powerful data-backed algorithms, it is increasingly possible to make veterinary decisions based on probability rather than a clinical diagnosis. This can help producers, veterinarians and animal care, but regulators have not accepted this as a valid method for justifying treatment.
- Data storage and confidentiality requirements – policies that require data be processed and/or stored in servers within a country make it difficult for digital services to be delivered at scale. In addition, data confidentiality and ownership requirements remain unclear in some market.
- Unclear regulatory situation – there are often no clear indications of how such products should be assessed. In addition, animal health regulators may lack the background to assess such products.
Opportunities to lift barriers to innovation
Digital technologies constitute new territory in the agricultural world. This brings challenges, but also vast opportunities to accelerate the transition to digital health.
- Effective data protection – for data-driven solutions to reach their full potential, it is crucial that regulators ensure fair and effective data protection for all stakeholders. This means making sure that animal caretakers are protected, while not stifling the potential of digital solutions.
- Rural connectivity – limited connectivity in many parts of the developed as well as the developing world is a major barrier to digital advancements. Improving access to telecommunications and internet in rural areas would help accelerate the uptake of digital innovations.
- Incentive schemes – if regulators, governments and the private sector can provide incentives to accelerate digital development and support infrastructure, the collective benefits can compound at a faster rate.
- Adapting regulations – if regulatory systems include well-being improvements as a recognized claim, not just health improvements, this could allow for more products that support better welfare.
Diagnostics
Overview
- Accurate diagnostics are imperative to curbing the severity of animal disease by enabling more effective treatments and reducing the need for antibiotics.
- A large share of diagnostic innovation is happening in the digital sphere where artificial intelligence and user-friendly tools are potential game-changers for the animal health industry.
- The development of diagnostic tools is stifled by limited veterinary infrastructure in rural areas which hampers adoption and the incentive for innovation.
- Strategic financial and regulatory support for technology can help increase the pace of innovation and strengthen the digital ecosystem.
The state of innovation
A selection of technologies reaching the market or on the horizon
Artificial intelligence
Artificial intelligence has the ability to improve diagnostics by enabling more predictable, rapid analysis of samples that can quickly and effectively identify disease. For example, AI algorithms can detect parasitic eggs in faecal samples, reducing the workload of veterinarians, who would normally need to compare sample slides for signs of parasites. This could provide greater accuracy over traditional, in-clinic analyses of samples that are subject to human error. Not only can this enable faster or even real-time results, it can also allow for predictive diagnoses via algorithms that determine the probability of animal disease.
Timeframe: Products are already on the market
Microfluidics
Microfluidic devices are tiny chips that can conduct analyses on extremely small volumes of fluids, such as blood. This enables practitioners to bring diagnostic devices to the point-of-care, which increases convenience for the user and improves the chances of early detection of disease. Since microfluidic devices can test smaller sample volumes than conventional tests, this also helps to reduce costs and turn-around time.
Timeframe: Products are already on the market
Molecular diagnostics
Molecular diagnostics refers to a collection of techniques for analysing biological markers in the genetic code and proteins of an organism. It is a promising field in veterinary medicine that is rapidly evolving. While these technologies exist in laboratories, many pharmaceutical actors are looking to bring this high-precision method to the point-of-care in the livestock industry to improve the accuracy of diagnostics.
Timeframe: Five to 10 years
Sustainability benefits
Improved diagnostics – remote as well as on-site – will help protect animals against severe disease which also means:
- Safeguarding the livelihoods of millions of people who rely on livestock (SDG 1, 2 and 8);
- Reducing the use of antibiotics and thus helping minimise potential antimicrobial resistance – a major threat to public health (SDG 3);
- Limiting the transmission of zoonotic diseases that spread between animals and humans (SDG 3).
Barriers to innovation
Developing more effective diagnostics cannot be done in a vacuum – limited infrastructure and high costs of adoption include some of the major barriers that stand in the way.
- High barriers to entry – New diagnostic tools need to be affordable and suitable to the end-user. Even if novel solutions help reduce costs in the long run, they must be appropriately priced and well-designed to attract early adopters.
- Limited connectivity – Digital tools require connectivity. Many rural areas in the Global South and the Global North alike suffer from limited access to internet, which limits adoption and thus also innovation.
- Shortage of veterinarians – There is a shortage of veterinarians in the marketplace. While technology enables practitioners to connect from across great distances, a shortage of staff makes it challenging to create thriving digital ecosystems.
Opportunities to lift barriers to innovation
Innovation in diagnostics could be accelerated through improved infrastructure and regulatory flexibility.
- Improving infrastructure – By strengthening rural infrastructure and increasing internet connectivity, more people will be able to use new tools as they reach the market and ultimately help create a strong digital community.
- Financial support – Without investment there is no innovation. Private or public financial support mechanisms that help attract investment into diagnostic tools – or subsidise their use – could play a major role in driving innovation.
- Enabling prediction-based diagnostics – Artificial intelligence will allow animal health practitioners to diagnose animals based on probability. This needs to be factored into regulations to ensure that treatment can be administered in lieu of a clinical diagnosis.
Parasite control
Overview
- Parasites are spreading to new regions as climate change allows them to survive in warmer temperatures, which poses a significant threat to both pets and livestock.
- New methods of control, administration and stewardship are improving parasite control while also managing the challenge of resistance.
- Regulation must keep pace with innovation and distinguish between human and animal health challenges to allow new products to reach the market.
The state of innovation
A selection of technologies reaching the market or on the horizon
Warming temperatures due to climate change is allowing parasites to thrive in new regions, increasing the urgency to discover new parasiticides and other methods of parasite control.
mRNA Parasite Vaccines
New vaccines that use mRNA technologies to control parasites could allow for more effective vaccines that can be reliably produced. Parasite vaccines developed through traditional methods (e.g. attenuated) are often difficult to reliably manufacture for companies. For example, parasites may need to be harvested for production, which poses challenges for standardization and quality control. Only a handful of parasite vaccines are available in veterinary medicines as a result. However, mRNA could allow for more reliable production as these can avoid the difficulties of traditional methods.
Timeframe: Five to ten years
Oral Parasiticides
Flea and tick control products that can be administered orally are a growing segment of the pet market and will likely expand to livestock in the future. Traditional parasiticides are administered through methods such as injection, topicals, and pour-on. These are effective but may require training or experience for proper application. Oral parasiticides can provide an easier, simpler and more convenient form of administration.
Timeframe: Products are already on the market
‘Green’ Parasiticides
Parasiticides that degrade quickly after use can limit entry into the environment and offer a ‘greener’ product profile. Parasite resistance is a concern in both animal and human health, therefore limiting entry of parasiticides into the environment is an important part of responsible use. Parasiticides that degrade in an animal before it is excreted, or shortly thereafter, can reduce the amount of active ingredient that may enter the environment.
Estimated Timeframe: Three to five years
Sustainability benefits
New methods for parasite control can help improve animal health, public heath, and manage resistance, which can deliver sustainability improvements such as:
- Greater productivity as a result of improved health (SDG1, 2 and 8);
- Better control of zoonotic, vector-borne diseases (SDG3);
- Reducing the potential impact of parasiticide use on the environment (SDG 12).
Barriers to innovation
Current and next-generation parasiticides can offer out-sized value if the right regulatory approaches are in place:
- Conflating human and animal health needs – Parasiticide resistance is often treated as a public health threat comparable to antibiotic resistance, despite mainly affecting animals. This can lead to unnecessary requirements to meet public health standards that are not wholly relevant, while parasiticides can face additional scrutiny from food safety agencies in markets where they may also be categorized as pesticides.
- Repetitive safety requirements – Regulators increasingly require evidence of a threat from all relevant parasites when assessing a new combination of active ingredients. This can delay the development of a treatment that requires multiple active ingredients if it cannot be demonstrated that all relevant parasites are a current threat.
- Product efficacy claim limitation – In certain markets, products that are effective against harmful parasites may be unable to claim this on labels due to regulatory limitations. For instance, claims against ‘invasive species’ are disallowed in one market even though these may pose a threat to animals, which can lead to off label product usage.
Opportunities to lift barriers to innovation
Recent developments in EU regulation have relieved some issues facing biologicals but regulators can further stimulate innovation by:
- Distinguishing between human and animal health needs – Antimicrobial and antiparasitic resistance represent different public health considerations and this should be considered in regulatory assessments.
- Streamlining safety assessments – By aligning the regulatory review of products like parasiticides across medical and food safety regulators, the approval process can be streamlined, saving time, money and resources.
- Recognizing zoonoses control – allowing parasiticides to include zoonotic disease control claims, after demonstrating efficacy through modeling, can strengthen development of products that directly improve public health
Nutrition
Overview
- Managing immunity and gut microbiome through nutrition offers enormous untapped potential for improving overall health.
- Products are already making an impact such as in reducing the need for antibiotics, however more research is needed into gut health to better understand this emerging area.
- Regulatory requirements are not always adapted to nutritional products as it is a new area of innovation with novel technologies.
The state of innovation
A selection of technologies reaching the market or on the horizon
Growing knowledge about the role of nutrition, gut health and natural immunity to disease is opening up new opportunities for improved animal health and welfare.
Probiotics
Pre- and pro-biotic molecules added to animal feed can prevent disease and target specific health challenges, such as mastitis in cows or vibriosis in aquaculture. Combining different elements in feed can provide multiple, direct benefits from increased immunity, reduced gut bacteria and a balanced digestive system.
Timeframe: Products already on the market
Phytogenic feed additives
Phytogenic, or plant-based, substances with anti-bacterial properties, are increasingly being identified, combined and added to animal feed to alter the gut microbiome, improve immunity and protect against specific diseases. Combining the different, natural and beneficial qualities of essential oils and plants like oregano, yucca, quillaja, garlic and mushroom can offer improvements in health that reduce the need for traditional antibiotics in both livestock and aquaculture. Research is under way to develop feed additives that can help reduce Salmonella and Campylobacter in poultry.
Estimated timeframe: One to five years
Novel feeds
Alternative animal feeds such as insect-based protein and seaweeds are opening opportunities for precision nutrition. Feeding animals according to stage of life, gut health and environmental factors offers benefits for health and welfare as well as sustainability and traceability. New products are being tested for palatability and impact on animal-sourced food.
Estimated timeframe: One to five years
Strengthening Natural Immunity
The microbiome of an animal helps it fend off harmful disease.
Special additives such as probiotics can strengthen the microbiome.
This supports an animal’s natural immune system, much like people take supplements.
Sustainability benefits
Improving animal health through precision nutrition and feed additives offer multiple benefits to animal health and wellbeing, as well as greater sustainability such as:
- Greater productivity as a result of improved health (SDG1, 2 and 8);
- Fewer resources needed and lower emissions (SDG12, 13 and 15).
Barriers to innovation
As an emerging field, nutritional supplements and technologies often face a regulatory landscape that has not fully adapted:
- Disincentivising the most advanced technology – developing a combination of synthetic compounds to manage specific diseases will be subject to more rigorous, costly and resource-intensive safety assessment than substances known to be safe but with generic health benefits. This discourages manufacturers from developing the most targeted, cutting edge products.
- Untapped potential – Gut health is an emerging area in animal health research that has grown rapidly in recent years. However, much like in human health, our understanding of the microbiome is still in early stages.
- Disparate Regulations – Requirements for registering a novel nutritional product can widely differ across various markets. This makes it difficult for a company, especially smaller ones, to generate the data required to enter enough markets for product viability.
Opportunities to lift barriers to innovation
Addressing roadblocks to nutrition innovation could be achieved through:
- Increased basic research – Greater investments in nutrition research by public institutions could accelerate our knowledge in emerging spaces such as gut health and lead to new breakthroughs that can strengthen animal health, reduce the need for antibiotics, improve sustainability, etc.
- Updating regulatory requirements in line with innovation – existing regulatory requirements are not always suitable for assessing novel nutrition technologies because not all criteria is relevant or applicable.
Safe development
Overview
- New animal medicines and products must be proven to be safe and effective before being approved, which relies on an extensive process of testing on live animals.
- In-vitro testing, stem cell technology and biomarkers are among the innovations allowing researchers to demonstrate safety and efficacy while reducing the need for animal testing.
- However, alternative testing methods must be shown to meet the same clinical standards as animal testing or risk being rejected.
- Developing new clinical standards for alternatives to animal testing could accelerate research and development.
The state of innovation
A selection of technologies reaching the market or on the horizon
Rigorous testing of new medical products on live animals raises concerns about animal welfare. Developing safer, quicker and less expensive alternatives for demonstrating safety and efficacy can reduce the cost of product development and limit the need for live animals.
Biomarkers
Biomarkers have the potential to demonstrate drug or treatment efficacy more quickly and accurately in both people and animals by measuring biological processes associated with specific diseases, rather than clinical outcomes such as disease progression or mortality. This allows some medicines to be evaluated without subjecting an animal to increased handling and stress. Examples of biomarkers include a change in blood pressure or the appearance of certain proteins.
Advances in scientific understanding of biomarkers can also help develop targeted treatments and measure their impact before full symptoms emerge. In some cases, biomarkers, such as genetic mutations showing a predisposition to cancer, are relevant to both human and animal health, creating an opportunity for cross-disciplinary collaboration to advance the “One Health” agenda that recognises the overlaps between the sectors.
Estimated timeframe: Up to 10 years
In-vitro testing and stem cell technology
Testing a new product on cells or tissue in a lab (in-vitro) is an emerging alternative to animal testing. Researchers are studying whether stem cells can be used to test vaccines against diseases. Scientists are also exploring whether genetic modification can provide an efficient source of white blood cells on which to test a potential vaccine candidate. These could help demonstrate a vaccine’s potential efficacy sooner without the need for animal testing.
Estimated timeframe: Up to 10 years
Measuring Reactions through Biomarkers
Animals can often suffer pain without showing any physical discomfort. However, the body will release ‘biomarkers’ (e.g. hormones or enzymes) that provide a reliable way to measure pain without waiting until an animal is visibly suffering. This allows for safer product testing.
Sustainability benefits
Developing ways to test new drugs or treatments that require fewer animals can protect animal welfare, and make product research and development safer and more sustainable through:
- Developing treatments faster and with more precision to protect agricultural livelihoods (SDG1 and 8);
- Sharing knowledge of biomarkers across human and animal health (SDG3);
- Reducing the cost and losses of live animals (SDG12 and 15).
Barriers to innovation
While technology for alternative testing is developing quickly, regulatory requirements maintain the same clinical indicators to demonstrate the effectiveness and safety of a product. This means companies must demonstrate that their alternative method of testing is as reliable as animal testing.
There is also a discrepancy between the standards of validation for biomarkers in pivotal studies, compared to those used as diagnostic tests by veterinarians.
Animal testing remains a requirement for many countries, so manufacturers must continue to use animal testing for any product intended to be marketed globally instead of alternative methods.
Case study: pain relief
Establishing a reliable and objective measurement of clinical indicators such as pain demonstrates the challenge facing both regulators and developers.
Companies have tried to quantify pain in cows, for example, through vocalization, to illustrate what pain and discomfort is, in order to demonstrate that pain relief medication is effective.
Solutions have included using force plates to measure lameness by indicating a limp because of pain.
Biomarkers could offer a more accurate and consistent metric by which to assess medicines.
Opportunities to lift barriers to innovation
Regulators and health agencies can facilitate more innovation in alternative testing methodology and safer product development through:
- Increasing public funding – investing in more research into animal-free testing through initiatives like the EU’s Horizon 2020 framework can help meet public health goals and consumer needs.
- Responsive regulation – developing new methodologies and clinical standards for alternative testing can foster more innovation in this field and move towards validating new, quicker and more accurate processes and reduce the need for research animals.
- Greater focus on 3Rs – The 3Rs framework seeks to reduce animal testing through ‘replacement, reductions and refinement’ of existing procedures. However, requirements such as unnecessary batch safety testing still exist in certain markets. Increased focus on implementing the 3Rs could help alternative methods emerge and reduce animal testing.
Conclusion
The speed with which several candidate vaccines against Covid-19 reached the final stages of development just months after the 2020 pandemic began was a testament to both the power of science and the ability of regulators to act quickly in the face of a global health emergency.
Yet the loss of one in five animals to disease every year is an ongoing and overlooked emergency that manifests around the world at varying rates and intensities. The consequences are far-reaching, starting with animal welfare and extending to economic growth, food security and human health and wellbeing.
There are many promising innovations on the horizon that could prevent animal diseases or diagnose and treat them quicker with greater accuracy, which means more food in the supply chain, greater income opportunities for farmers and better health prospects for consumers and their pets.
From heat sensors to monitor for signs of fever to feed supplemented with health-boosting ingredients and stem cell technology that could offer a way to reduce the number of animals needed for testing, scientific advances offer enormous potential for better animal health.
And all of these innovations contribute to minimising the threat of antibiotic resistance to public health by reducing the need for antibiotics in animals.
Smoothing the route to bringing these new and emerging solutions to market will unlock benefits of improved animal health, making raising animals more sustainable. Achieving this will require action in four key areas:
- Develop more responsive, agile and consistent regulation that allows manufacturers to respond to new and changing disease threats without needing to repeat safety assessments for existing technologies, avoids unnecessary data generation and adapts for new categories of products.
- Consider new regulatory approaches in regions where market size may be a barrier to entry, such as regulatory harmonization or considering approvals by trusted trading partners as a criteria for fast-tracking a product assessment.
- Provide sufficient data protection and avoid trade barriers, allowing companies adequate opportunity to recoup the time, resources and financing needed to bring a product to market, which would incentivise further investment.
- Ensure strong economic incentives, including public financing, that strengthen the contribution of animal health to public health. This might include financial incentives for farmers to adopt vaccines that provide societal benefit or market entry rewards for antibiotic alternatives.
Facilitating development and pathways to market is essential to deliver the next generation of innovation in animal health. The benefit will be a healthier, more sustainable world.